While change of sensitivity in FI mode requires a physical reconfiguration of a manifold, in microSI mode the change of calibration range is achieved by means of computer control by programming:
- sample and reagent volumes
- incubation time (stop flow period)
- position of the data collection window.
The high sampling frequency, and lower sensitivity (A,B,C) is obtained by using smaller sample and reagent volumes and by eliminating stop time in the holding coil (protocol C).
Higher sensitivity, at a lower sampling rate (D,E,F) is obtained by injecting larger sample and reagent volumes and by including a 10 second incubation time (WAIT).
Both protocols include data collection window (yellow shaded interval shown in superimposed calibration runs A,D) which is set in order to identify the position of a peak, to be used for automated construction of calibration graph (B,E).
Note: Data collection and formation of calibration graph in real time, slows performance of the software. Therefore the actual sampling rate of a calibration run is lower than 150s/hr, that corresponds the sample cycle of 24 seconds (A).
The point is, that it is not the rate of chemical reactions, or efficiency of microfluidics, but the software script that needs to be upgraded, should higher sampling frequencies become desirable.
Nitrite Assay
0 to 200 ppB N and 0 to 50 ppB N
2.2.18.
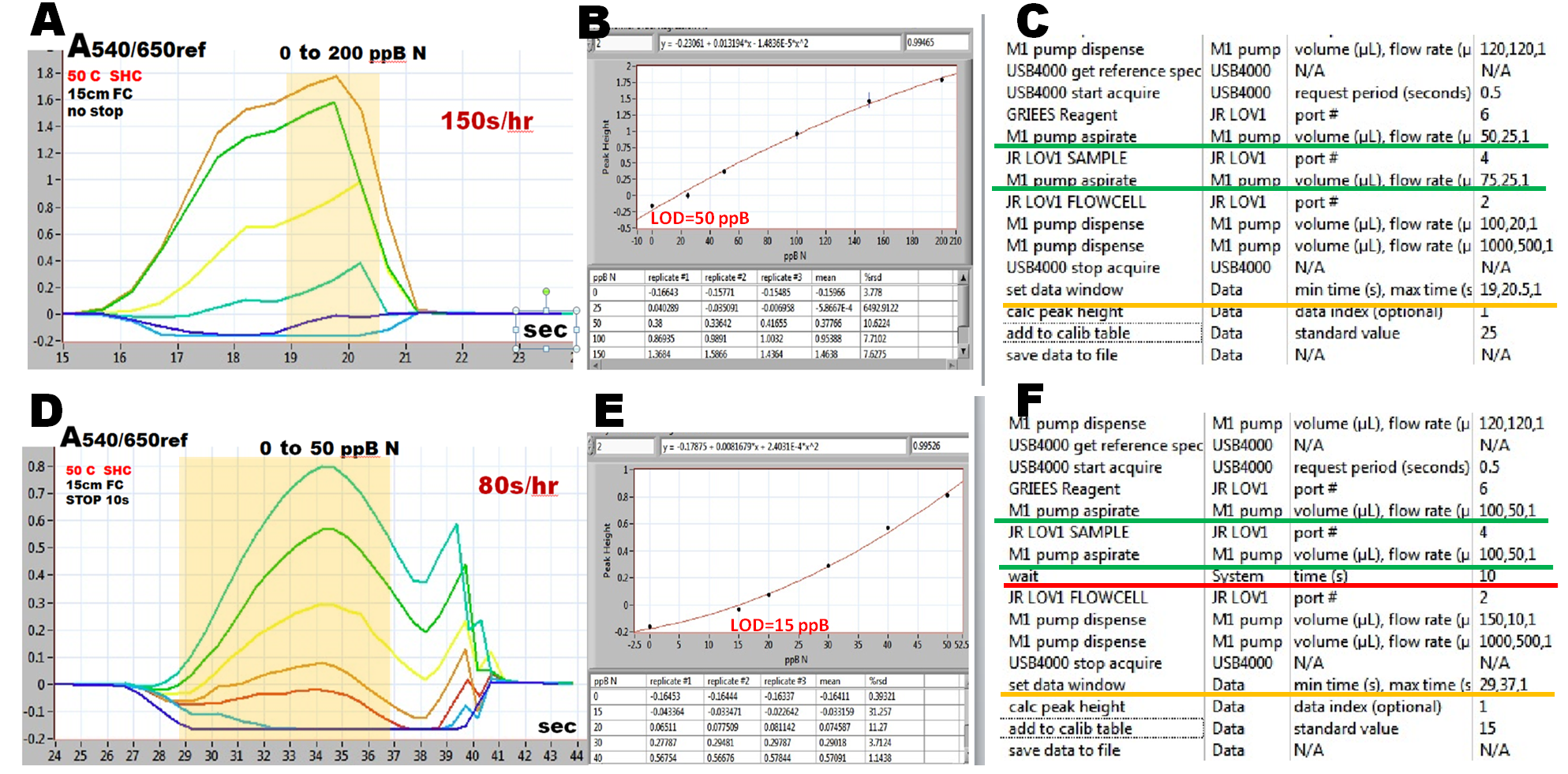
This assay protocol is a milestone in the development of Sequential Injection assays for two reasons. First this is the highest sampling frequency achieved by SI technique. Next, this assay protocol can serve as a template for all single reagent assays. It is remarkable how little is known about kinetics of the commonly used reagent based assays. As shown in this section, assays of nitrate, phosphate and ammonia can be easily accelerated beyond what has been an accepted norm, by using efficient mixing by rapid flow reversal and by accelerating reaction rate by means of elevated temperature.









